Description
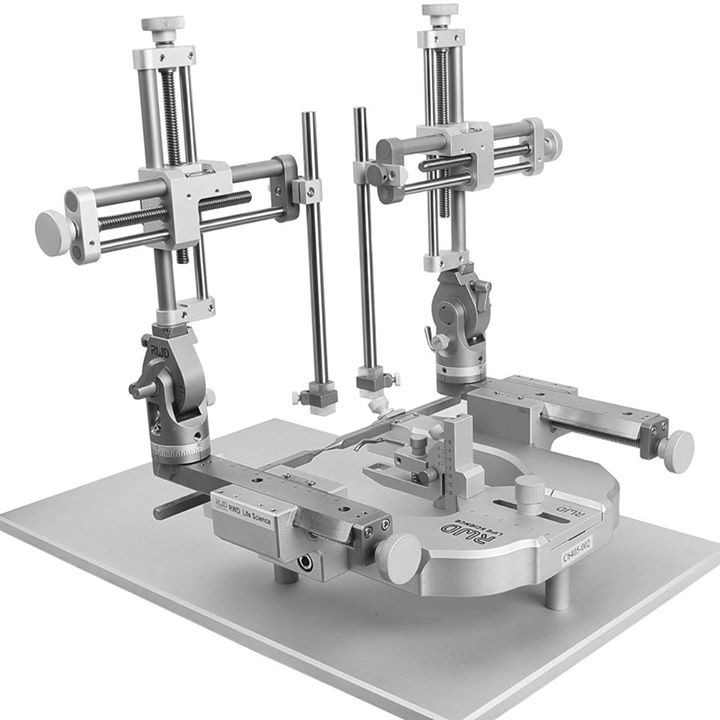
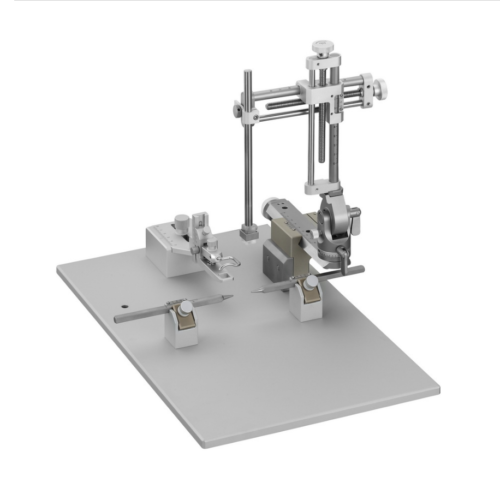
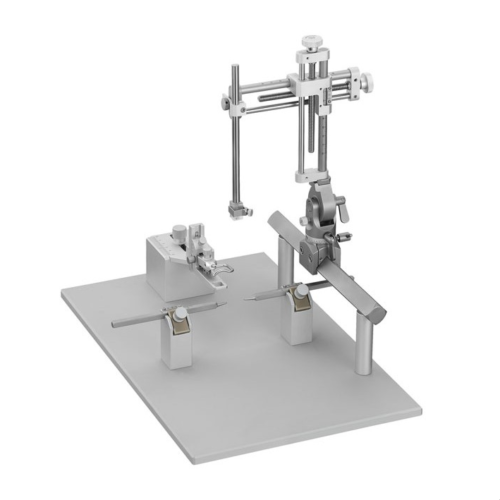
Cadre stéréotaxique pour la chirurgie crânienne lesté avec un support en U et livré pour la chirurgie sur rongeurs anesthésiés (Modèle VI-688xx de base).
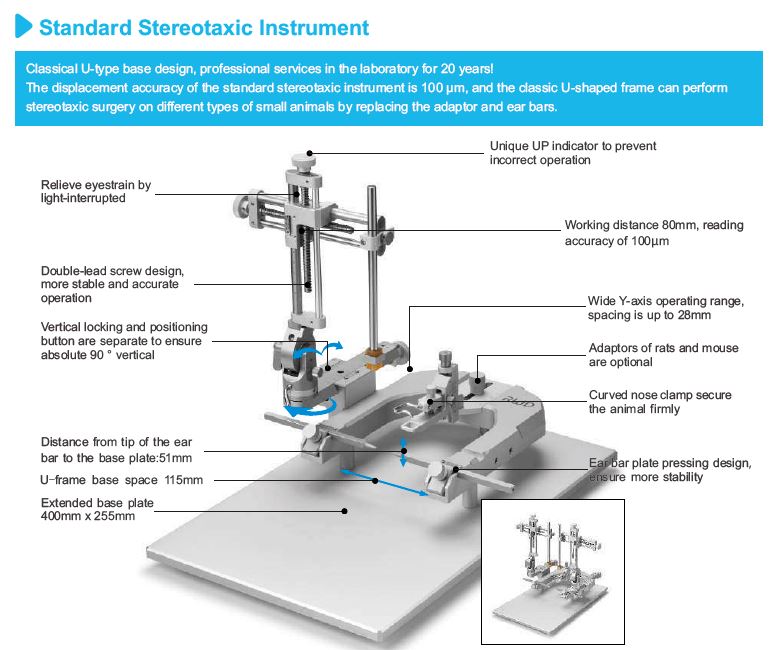
Une base surmontée d’un corps massif en U, un (ou deux) micromanipulateur à 3 axes de déplacement équipés de verniers (lecture sur graduation laser, rés. 100 microns).
Barres d’oreille, adaptateur de gueule et porte accessoire sont à choisir séparément:
Barre de gueule ici
Barres d’oreille ici
Portes- accessoires ici
Applications typiques
- Injection stéréotaxique souris, de nouvelles formulations médicamenteuses, molécules inhibiteurs et cultures cellulaire
- Mise en place de Micro-dialyse cérébrale
- Placement pour Stimulation nerveuse et enregistrement du signal EEG
- Optogénétique neurophotométrie ( FP)
- Imagerie cérébrale in vivo
- Modélisation des lésions cérébrales et de la moelle épinière
——–
Avec les accessoires adéquats, ce cadre peut être utilisé avec d’autres espèces après acquisition.
La gravure des verniers est travaillée de manière à réduire la fatigue visuelle. Des icônes dé-trompeuses permettent de prendre en main le sens de positionnement rapidement.
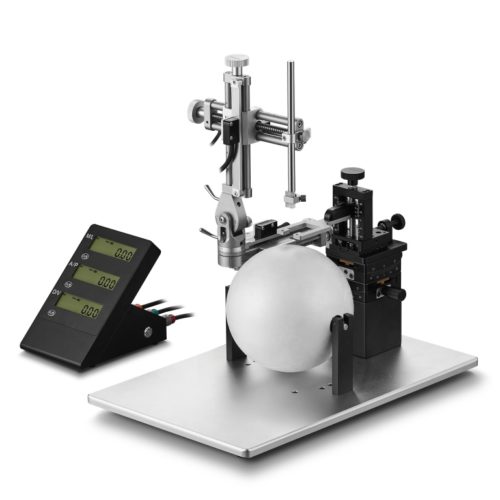
Ces tables stéréotaxiques avec support en U et micromanipulateurs à verniers à lecture analogique peuvent être mise à niveau avec un kit digital comprenant les capteurs de position ( résol. augmentée à 0,01 mm) et un afficheur déporté.
Remarque:
la configuration de base est évolutive et peut accueillir un deuxième micro-manipulateur plus tard sous forme d’un kit à monter. De nombreux accessoires d’aide à la chirurgie peuvent s’adapter au cadre, poursuivez votre découverte du site ou si vous avez un doute, contactez nous.
Publications
The Distribution of Transplanted Umbilical Cord Mesenchymal Stem Cells in Large Blood Vessel of Experimental Design With Traumatic Brain Injury
Dong, Hua-Jiang et al. Journal of Craniofacial Surgery 2017
The authors aim to track the distribution of human umbilical cord mesenchymal stem cells (MSCs) in large blood vessel of traumatic brain injury -rats through immunohistochemical method and small animal imaging system. After green fluorescent protein (GFP) gene was transfected into 293T cell, virus was packaged and MSCs were transfected. Mesenchymal stem cells containing GFP were transplanted into brain ventricle of rats when the infection rate reaches 95%. The immunohistochemical and small animal imaging system was used to detect the distribution of MSCs in large blood vessels of rats. Mesenchymal stem cells could be observed in large vessels with positive GFP expression 10 days after transplantation, while control groups (normal group and traumatic brain injury group) have negative GFP expression. The vascular endothelial growth factor in transplantation group was higher than that in control groups. The in vivo imaging showed obvious distribution of MSCs in the blood vessels of rats, while no MSCs could be seen in control groups. The intravascular migration and homing of MSCs could be seen in rats received MSCs transplantation, and new angiogenesis could be seen in MSCs-transplanted blood vessels.
Enhanced Autophagy Contributes to Protective Effects of GM1 Ganglioside Against Aβ1-42-Induced Neurotoxicity and Cognitive Deficits
Ruwei Dai et al. Neurochemical Research 2017
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder. The aggregation of Aβ peptides, Aβ1-42 in particular, is thought to be a fundamental pathogenic mechanism leading to the neuronal damage in AD. Recently, monosialoganglioside GM1 is reported to possess pivotal neuroprotection in neurodegenerative diseases. Previous studies have focused on the conformational dynamics and the biochemical interaction of the amyloid-peptide with the GM1 ganglioside, as well as the protective effect of GM1 on cognition. However, the phenomenon of autophagy with regard to neuronal dysfunction in AD is less investigated. In the present study, GM1 treatment were investigated in an AD mouse model and cultured PC12 dells to examine cognition-protective and neuroprotective effects of GM1. Furthermore, GM1 was found to induce autophagy via testing light chain 3 (LC3), Beclin1, neighbor of BRCA1 gene 1 protein and p62 (a substrate of LC3). Chloroquine, an inhibitor of lysosomal, was used to exclude the interference of lysosome, which could fuse with autophagosome and then clear it. In the presence of the inhibitor of autophagy (3-methyladenine; 3-MA), the protective effect of GM1 on PC12 cells in Aβ (1-42) induced toxic conditions was diminished. Interestingly, the expression of histone deacetylase 1 was increased in PC12 cells when treated with GM1, indicating that autophagy might be activated by GM1 through a pathway integrates protein acetylation. This study provides a novel insight into the protective role of GM1 against Aβ (1-42)-induced neurotoxicity via enhancing autophagy.
Influence of intramuscular heat stimulation on modulation of nociception: complex role of central opioid receptors in descending facilitation and inhibition
Hao‐Jun You et al. 2014. the journal of physiology
It has been reported that the threshold to activate ‘silent’ or inactive descending facilitation of nociception is lower than that of descending inhibition. Thus, the development of pain therapy to effectively drive descending inhibition alone, without the confounding influences of facilitation is a challenge. To address this issue we investigated the effects of intramuscular stimulation with a heating‐needle on spinal nociception, assessed by measuring nociceptive paw withdrawal reflex in rats. Additionally, involvement of the thalamic ‘nociceptive discriminators’ (thalamic mediodorsal (MD) and ventromedial (VM) nuclei), and opioid‐mediated mechanisms were further explored. Descending facilitation and inhibition were elicited by 46°C noxious heating‐needle stimulation, and were regulated by thalamic MD and VM nuclei, respectively. In contrast, innocuous heating‐needle stimulation at a temperature of 43°C elicited descending inhibition modulated by the thalamic VM nucleus alone. Microinjection of μ/δ/κ‐opioid receptor antagonists β‐funaltrexamine hydrochloride/naltrindole/nor‐binaltorphimine, into the VM nucleus attenuated the 46°C intramuscular heating‐needle stimulation‐evoked descending inhibition, whereas treatment of the MD nucleus with β‐funaltrexamine hydrochloride significantly decreased the descending facilitation. By contrast, descending inhibition evoked by 43°C heating‐needle stimulation was only depressed by naltrindole, as opposed to μ‐ and κ‐opioid receptor antagonists, which failed to influence descending inhibition. The present study reveals distinct roles of μ‐opioid receptors in the function of thalamic MD and VM nuclei, which exert facilitatory and inhibitory actions on nociception. Furthermore, innocuous, but not noxious, intramuscular heating‐needle stimulation targeting δ‐opioid receptors is suggested to be a promising avenue for the effective inhibition of pain.